RADICUT Oral Suspension 2.1% for amyotrophic lateral sclerosis (ALS, edaravone, Radicava)
What is RADICUT® Oral Suspension 2.1% for amyotrophic lateral sclerosis (ALS, edaravone, Radicava)?
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative condition characterized by the gradual deterioration of motor neurons, resulting in muscle weakness and wasting across various parts of the body, including limbs, facial muscles, and the respiratory system. The exact cause of ALS remains largely unidentified, although factors such as genetics and environmental influences are believed to play a role. With a global annual incidence rate of approximately two cases per 100,000 individuals, ALS ranks among the most prevalent neuromuscular diseases worldwide.
For individuals seeking treatment options for ALS, RADICUT® Oral Suspension represents a significant therapeutic breakthrough. Developed by Mitsubishi Tanabe Pharma Corporation, RADICUT® Oral Suspension offers ALS patients a convenient and effective alternative. Its active ingredient, edaravone, a potent free radical scavenger, was first approved in Japan in 2001 for treating acute cerebral infarction and later endorsed for ALS treatment in multiple countries, including the United States, Canada, and Switzerland. The availability of edaravone in an oral suspension form alleviates the challenges associated with intravenous administration, such as injection-related discomfort and frequent hospital visits.
How does it work?
The Mechanism of RADICUT® Oral Suspension involves delivering consistent therapeutic benefits comparable to its intravenous counterpart. Administered once daily in a 5 ml dose using an oral dosing syringe, this formulation ensures ALS patients receive essential medication without the inconvenience of injections. This approach not only enhances treatment adherence but also contributes to a better quality of life for patients managing ALS.
Scientific studies validate the efficacy of RADICUT® Oral Suspension. A bioequivalence study comparing the oral suspension to the intravenous form demonstrated similar plasma concentration profiles, with the oral suspension achieving even higher levels of edaravone's inactive metabolites. Both formulations exhibited comparable urinary excretion patterns, underscoring the oral suspension's equivalence in delivering therapeutic benefits compared to its intravenous counterpart.
RADICUT® Oral Suspension represents a significant advancement in ALS management, offering a user-friendly administration method that upholds the proven efficacy of edaravone. This innovation not only simplifies the treatment process but also provides ALS patients with a reliable therapeutic option supported by robust scientific evidence.
Active principles: edaravone
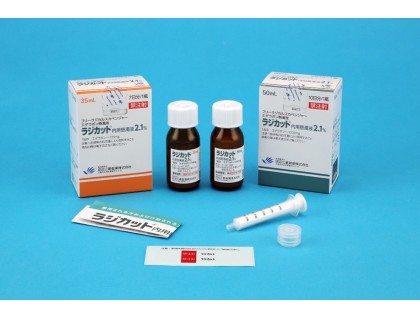
Amount: 1 bottle * 35 ml
Indications: suppression of progression of functional impairment in amyotrophic lateral sclerosis (ALS)
How to take: in general, for adults, 5 ml (105 mg of edaravone) is orally administered once daily on an empty stomach.
Normally, one course consists of 28 days of administration and rest periods, and this is repeated. In the first course, the drug is administered every day for 14 days, followed by a 14-day rest period, and from the second course onwards, 10 days of administration out of the 14 days are administered, followed by a 14-day rest period.
Contraindications and precautions: Store in a dry cool place, out of reach of children. Do not use for pregnant or breastfeeding women. Do not use for the patients with severe renal insufficiency. Not recommended for use in patients with ALS severity classification of grade 4 or higher and patients with forced vital capacity reduced to less than 70% of the theoretical normal value.
Since the plasma concentration of this drug decreases due to the influence of food, this drug should be taken after fasting for 8 hours, such as upon waking up, and food and drink other than water should be avoided for at least 1 hour after taking the drug. If fasting for 8 hours is not possible, this drug can be taken at least 4 hours after ingesting a low-fat meal or at least 2 hours after ingesting a light meal. However, this drug should be taken at least 8 hours after ingesting a high-fat meal.
Do not expose to sunlight or heat. If allergic symptoms occur, discontinue use.